Contact Us
Edward T. Morgan, PhD
Professor
Dept. of Pharmacology and Chemical Biology
Emory University School of Medicine
1510 Clifton Road
5th Flooor
Emory University
Atlanta, GA 30322
Tel (404) 727-5986
Fax (404) 727-0365
etmorga@emory.edu
Research in the Morgan Lab
The Morgan lab is interested in the regulation of drug metabolizing enzymes in disease states, with an emphasis on cytochrome P450 enzymes and inflammatory and infectious diseases.
IN VIVO DISEASE MODELS
The majority of P450 enzymes are down-regulated in the liver during inflammation or infection, and most of these changes are thought to be due to the hepatic actions of proinflammatory cytokines. Different cytokines regulate different P450s, and limited information suggests that the pattern of P450 regulation, and hence alterations in drug metabolism and clearance, are disease-dependent.
One of the goals of the lab is to further define the complexity of this regulation in different disease models. We have extensively characterized a model of food poisoning mice infected with Citrobacter rodentium. Our data show that tumor necrosis factor-a is responsible for the down-regulation of some Cyp3a enzymes. However, the hepatic CYP enzymes most affected in this disease are Cyp4as, which are more important in fatty acid metabolism than in drug biotransformation. Interestingly, Cyp4a10 and Cyp4a14 mice show reduced systemic and hepatic inflammation during infection, implicating these enzymes in the host response to infection. Current research is focused on two parasitic infections, malaria and schistosomiasis.
NITRIC OXIDE AND PROTEIN DEGRADATION
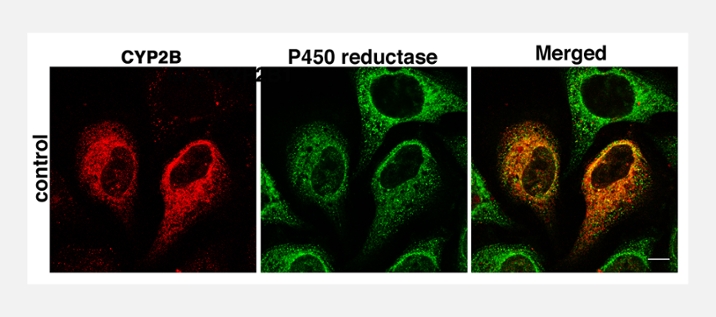
While many or even most of the effects of inflammation and cytokines on P450 activity and expression can be explained by regulation of gene transcription, we discovered that three rat liver enzymes, CYP2B1, CYP3A1 and CYP2C22 undergo stimulated protein degradation that is much faster than the transcriptional effects. This degradation is nitric oxide dependent, and occurs via the ubiquitination-proteasomal pathway. We are currently studying the effects of NO on human liver P450 enzymes, with a focus on CYP2B6. We want to understand the mechanism of this NO-regulated degradation, its importance for drug metabolism in vivo, and its applicability to other P450 and non-P450 proteins.